


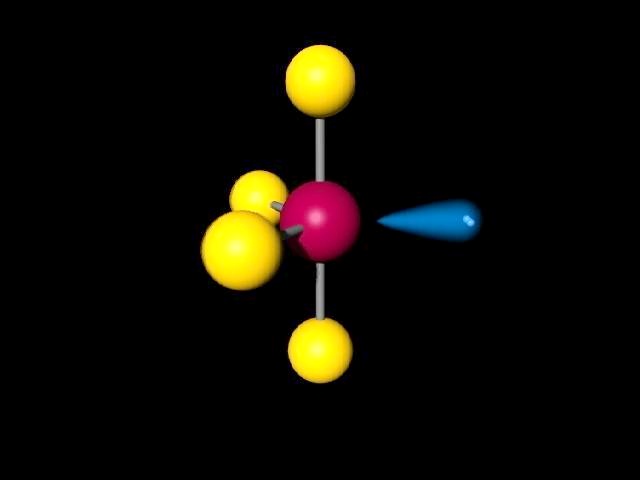
Some molecular compounds that adopt square pyramidal geometry are XeOF 4, and various halogen pentafluorides (XF 5, where X = Cl, Br, I). Seesaw or disphenoidal is a type of molecular geometry where there are four bonds to a central atom with overall C2v symmetry. Predict all bond angles for these molecules. CHCl3 (tetrahedral with C in the center position) arrowforward. CS2 (linear with C in the center position) d. NF2Cl (trigonal pyramidal with N at the apex) c. The electrons in the outer shell of an atom are involved in bonding. Chapter 8.Molecular Geometry and Bonding Theories (Homework) W Multiple Choice Identify the choice that best completes the statement or answers the question. An alternative geometry for four-coordinate metal centers that has rarely been observed is a seesaw geometry. NF3 (trigonal pyramidal with N at the apex) b. Chapter 8.Molecular Geometry and Bonding Theories (Homework) W CHEM 1411. The mechanism used is similar to the Berry mechanism. The molecular geometry is given in parentheses.
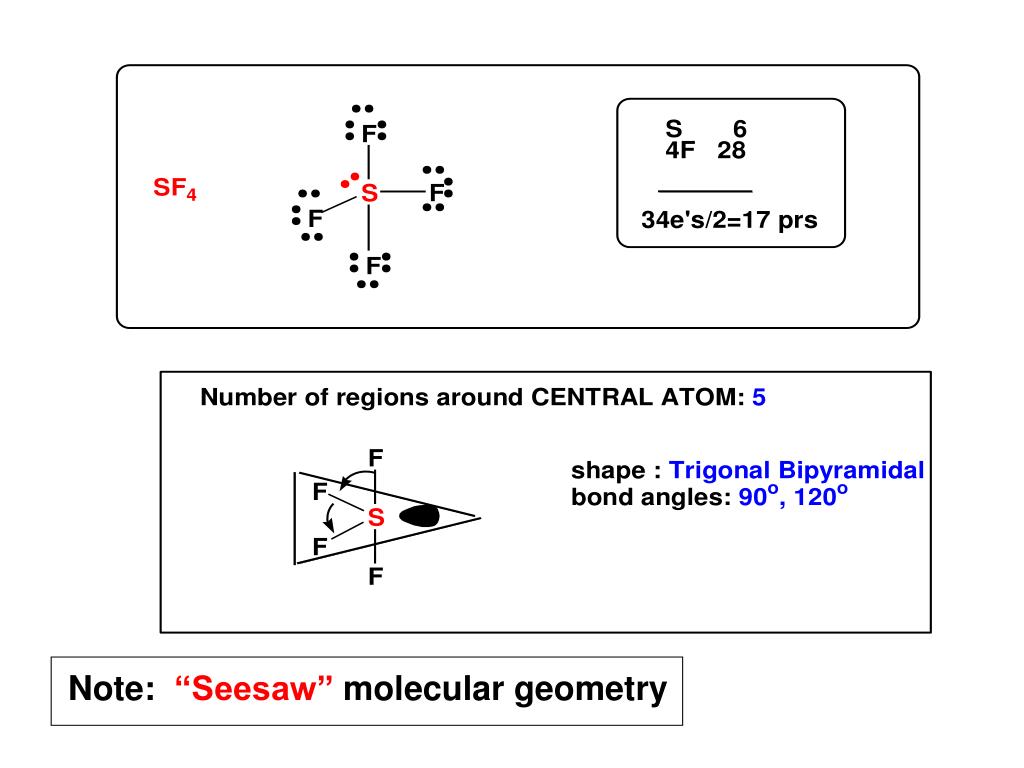
Molecules with this geometry, as opposed to trigonal bipyramidal, exhibit heavier vibration. of a tetrahedron are equivalent to one another this is not the case with the five. Seesaw is one of the molecular geometries of an atom. Pseudorotation also occurs in square pyramidal molecules. Name the molecular geometry from the atom positions. Thus even though the geometry is rarely seen as the ground state, it is accessed by a low energy distortion from a trigonal bipyramid. As a transition state in Berry pseudorotation Īs a trigonal bipyramidal molecule undergoes Berry pseudorotation, it proceeds via an intermediary stage with the square pyramidal geometry. Certain compounds crystallize in both the trigonal bipyramidal and the square pyramidal structures, notably 3−. In chemistry, a pentagonal bipyramid is a molecular geometry with one atom at the centre with seven ligands at the corners of a pentagonal bipyramid.A perfect pentagonal bipyramid belongs to the molecular point group D 5h. The geometry is common for certain main group compounds that have a stereochemically-active lone pair, as described by VSEPR theory. Structure of iodine heptafluoride, an example of a molecule with the pentagonal-bipyramidal coordination geometry. Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries. Well use the example of ClF3 to understand the molecular shape. The point group symmetry involved is of type C 4v. Sulfur tetrafluoride is the chemical compound with the formula S F 4.It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. In this video we’ll look at the T-Shaped Molecular Geometry and Bond Angles.
If the ligand atoms were connected, the resulting shape would be that of a pyramid with a square base. Square pyramidal geometry describes the shape of certain chemical compounds with the formula ML 5 where L is a ligand. Once again, we have a compound that is an exception to the octet rule. 10 Q What is the molecular shape of IF5 a) square pyramidal b) tetrahedral c) seesaw d) trigonal bipyramidal e) octahedral. Each fluorine has three lone pairs, Bromine has two lone pairs. Three fluorines are bonded to a central bromine. Structure of xenon oxytetrafluoride, an example of a molecule with the square pyramidal coordination geometry. The bromine atom has seven valence electrons, and each fluorine has seven valence electrons, so the Lewis electron structure is. 2 that the arrangement that minimizes repulsions places the groups 180 apart. There are two electron groups around the central atom. Shape of certain five-ligand chemical complexes Square pyramidal molecular geometryĬhlorine pentafluoride ( ClF 5), MnCl 2− 5 The central atom, beryllium, contributes two valence electrons, and each hydrogen atom contributes one.


 0 kommentar(er)
0 kommentar(er)
